Bihar Board 12th Chemistry Numericals Important Questions with Solutions in English
बिहार बोर्ड इंटर परीक्षा 2022 के सभी विद्यार्थी के सभी विषय की सभी प्रकार के प्रश्न का प्रारूप और PDF वर्ग नोट विषयवार सभी प्रकार के study note ( MCQ , Short question long question )![]()
Bihar Board Class 10 & 12 Science all subject Note and PDF
Question 1.
The decomposition of H2O2 was studies by titrating is at different intervals of time with KMnO4 Calculate the velocity constant for it from the following data, if the reaction is of the first order.
![]()
Solution:
The first order rate expression is :
K = \(\frac{2 \cdot 303}{t} \log \frac{a}{a-x}\)
The amount of KMnO4 is proportional to the amount of H2O2 present., so the volume of KMnO4 used at zero time corresponds to initial concentration (a) and the volume used after time, corresponds to (a-x) at that time. Inserting these values in the above equation, we get-
K600 = \(\frac{2 \cdot 303}{600} \log \frac{22 \cdot 3}{13 \cdot 8}\)
= 0.000837sec-1
K1200 = \(\frac{2 \cdot 303}{1200} \log \frac{22 \cdot 8}{8 \cdot 2}\)
= 0.000852 sec-1
The average value of velocity constant.
K = \(\frac{0.000837+0.000852}{2}\)
= 0.000844 sec-1
Question 2.
The optical reaction of sucrose in presence of dil HCl at various intervals is given in the following table:
![]()
Show that the reaction is of the first order.
Solution:
The inversion of sucrose will be a first order reaction if the above date confirms to the equation:
K = \(\frac{2 \cdot 303}{t} \log \frac{a}{(a-x)}\)
\(=\frac{2 \cdot 303}{t} \log \left(\frac{r_{0}-r_{\infty}}{n-r_{\infty}}\right)\)
Where, r0, r1 and r∞ are the optical rotation and the start of the reaction, after t and at the completion of rerion respectively.
Inserting the values in the above equation, we get-
K10 = \(\frac{2 \cdot 303}{10} \log \frac{32 \cdot 4-(-14 \cdot 1)}{28 \cdot 8-(-14 \cdot 1)}\)
\(=\frac{2 \cdot 303}{10} \log \frac{46 \cdot 5}{42 \cdot 9}\)
= 0.008060min-1
K20 = \(\frac{2 \cdot 303}{20} \log \frac{32 \cdot 4-(-14 \cdot 1)}{25 \cdot 5-(-14 \cdot 1)}\)
\(=\frac{2 \cdot 303}{20} \log \frac{46 \cdot 5}{39 \cdot 9}\)
= 0.008037min-1
K40 = \(\frac{2 \cdot 303}{40} \log \frac{32 \cdot 4-(-14 \cdot 1)}{19 \cdot 6-(-14 \cdot 1)}\)
\(=\frac{2 \cdot 303}{40} \log \frac{46 \cdot 5}{33 \cdot 7}\)
= 0.008054min-1
K100 = \(\frac{2 \cdot 303}{100} \log \frac{32 \cdot 4-(-14 \cdot 1)}{6 \cdot 7-(-14 \cdot 1)}\)
\(=\frac{2 \cdot 303}{100} \log \frac{46 \cdot 5}{20 \cdot 8}\)
= 0.008054min-1
The constancy in the value of K shows that the reaction is of first order.
Question 3.
Decomposition of diazobenzene chloride was followed at constant temperature by measuring the volume of nitrogen evolved at different times.
The date is given below
![]()
Calculate the specific reaction rate and order of reaction
Solution:
Diazobenzene chloride decomposition as:
C6H5 – N = N-Cl → C6H5Cl + N2
Thus the amount of N2 evolved will be a measure ofdiazobenzene chloride decomposed i.e.x. The total N23 evolved at infinite time will thus give the initial concentration i.e. a. Now substituting the values in the first order rate
expression,
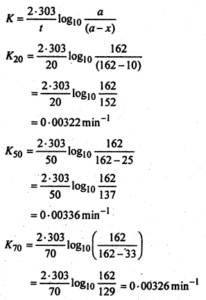
The constancy in the value of K shows that the reaction ¡s of the first order. The specific rate constant K is thus given by:
K = \(\frac{0.00322+0 \cdot 00336+0 \cdot 00326}{3}\)
= 0.00328min-1
12th Chemistry Numericals Important Questions with Solutions
Question 4.
A first order reaction is 15% complete in 20 minutes. How long will it take to be 60% complete?
Solution:
For a first order reaction, we have-
K = \(\frac{2 \cdot 303}{t} \log _{10} \frac{a}{(a-x)}\)
or,
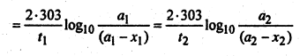
Here x1 =\(\frac{15}{100}\) a1
= 0.15a1,t1 = 20
x2 = \(\frac{60}{100}\)a2
= 0.6a2,t2 = ?
Inserting these values in equation (1), we get-
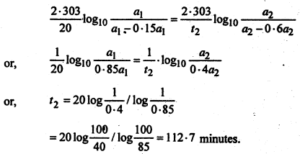
Question 5.
Given that the temperature co-efficient for the saponification of ethyl acetate by NaOH is 1.75. Calculate the activation energy.
Answer:
Given that-
\(\frac{K_{2}}{K_{1}}\) = 1.75 T1= (25 +273) = 298 K T2 = (35 + 273) = 308 K
(Since temperature co-efficient is ration of rate constant at 35° and 25°C respectively).
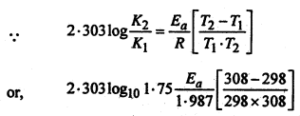
Ea = \(\frac{2.303 \times 298 \times 308 \times 1.987}{1}\) log 1.75 cal mol-1
or,
Ea = 10.207k cal mol-1
Colligative Properties of Solutions
Osmotic Pressure
Question 6.
Calculate the value of R in litre-atomosphere, if the osmotic pressure of a solution containing 45g. of sucrose dissolved per liter of solution at 2° C is 3.0 atmospheres.
Solution:
The mol wt. (m) of sucrose (C12H22O11)
= 12 × 12 + 1 × 22 + 16 × 11
= 342
T = 2 + 273
= 275 K
We known that p = \(\frac{w R T}{m v}\)
or,
R = \(\frac{P . m \cdot V}{w T}\)
R = \(\frac{3 \times 342 \times 1}{45 \times 275}\)
= 0.0829 lit-atm.
12th Chemistry Numericals Important Questions with Solutions
Question 7.
Calculate the osmotic pressure of 5% solution of glucose C6 H12O6 at 18° C.
Solution:
Given that, T = ( 18+273) = 291 K, w = 5g
V = 100ml = \(\frac{1}{10}\) = 0.1L and mg glucose = 180
we know that P = \(\frac{\boldsymbol{w}}{\mathrm{m} \cdot \boldsymbol{V}}\)RT
or,
p = \(\frac{5}{180 \times 0 \cdot 1}\) × 0.0821 × 291
= 6.636 atmosphere.
Question 8.
A solution containing 8 g of a substance per 100 ml was found to have an osmotic pressure of 500 cms of Hg at 27°C. Calculate the molecular weight of the substance.
Solution:
Given that, w = 8g, V= 100 ml = 0.1 L
\frac{500}{76}P = 500 cm = \(\frac{500}{76}\) atmosphere
and T = (27 + 273) = 300 K
We know that, p = \(\frac{w}{m V}\)RT
or,
m = \(\frac{w R T}{p . V}\)
\(=\frac{2 \times 0.0821 \times 300 \times 76}{500 \times 0.1}\)
= 299.4
Question 9.
A 1.02 % solution of glycerine is isotonic with 2% solution of glucose. What is the molecular weight of glycerine?
Solution:
For isotonic solution we have
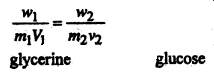
or,
\(\frac{1 \cdot 02}{m_{1} \times 0.1}=\frac{2}{180 \times 0.1}\) [∵ Mol. wt. of glucose = 180]
∴ m1 = \(\frac{180 \times 0.1 \times 1.02}{2 \times 0.1}\)
= 91.8
Question 10.
A solution containing 8.6 g/litre of urea (mol. wt. = 60) was found to be isotonic with a 5% solution of an organic solute. Calculate the molecular weight of the solute.
Solution:
For isotomic solution, we have-
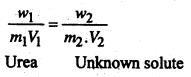
or,
\(\frac{8.6}{60 \times 1}=\frac{5}{m_{2} \times 0.1}\)
or,
m2 = \(\frac{60 \times 5 \times 1}{8.6 \times 0.1}\) = 348.9
Question 11.
10 g of a substance dissolved in 100 ml of water gave rise to an osmotic pressure 500 cm of Hg at 27°C. Calculate the molecular weight of the substance.
Solution- We know that-
m = \(\frac{w R T}{P V}\)
Given that, w = 4.0 ; P = \(\frac{500}{76}\)atm; V = 0.1 litre
R = 0.082 1 and T=(27+273) = 300 K
∴ m = \(\frac{4 \times 0.082 \times 300 \times 76}{500 \times 0.1}\) = 149.5
Question 12.
Calculate the osmotic pressure of \(\frac{\boldsymbol{M}}{\mathbf{1 0}}\) solution of cane sugar at 27° C.
Solution:
Molecular wt. of cane sugar (C12 H22 O11) = 342
For \(\frac{\boldsymbol{M}}{\mathbf{1 0}}\) solution, it means that \(\frac{1}{10}\) g mole of cane sugar is dissolved in 1 litre of solution i.e. =\(\frac{342}{10}\)g = 34.2 of cane sugar dissolved in 1 litre.
∴ W = 34.2g,V = 1 litre.
T = 27+273 = 300K
and m = 342
we know that P = \(\frac{w R T}{m V}\)
\(=\frac{34.2 \times 0.0821 \times 300}{342 \times 1}\)
or,
P = 2.463 atmosphere.
Question 13.
Calculate the amount of urea dissolved per litre if its equeour Solution is isotonic with 10% cane sugar. Molecular weight of urea = 60.
Solution:
For urea = m1 = 60; V1 = 1 litre, w1 = ?
For cane sugar = w2 = 10 g; m2 = 342, V2= 0.1 lit.
For isotonic solution:
\(\frac{w_{1}}{m_{1} V_{1}}=\frac{m_{2}}{m_{2} V_{2}}\)
or,
\(\frac{w_{1}}{60 \times 1}=\frac{10}{342 \times 0.1}\)
or,
w1 = \(\frac{10 \times 60 \times 1}{342 \times 0.1}\)
= 17.6 g/litre
Question 14.
At 298 K, 100 ml of a solution containing 3.002g of an unidentified solute exhibts an osmotic pressure of 2.25 atm. What is the molecular mass of the solute.
Solution:
Given that V = 100 ml = 0.1 litre, w = 3.002g, m = ?
T = 298 K,P = 2.25 atm and R = 0.0821
We know that
m = \(\frac{w R T}{P V}\) = \(\frac{3.002 \times 0.0821 \times 298}{2.25 \times 0.1}\) = 288.6
12th Chemistry Numericals Important Questions with Solutions
Lowering of Vapour Pressure
Question 15.
A current of dry air was drawn through solution of 3.5 g of a ubstance in 100g of ethyl alcohol and then passed through ethyl alcohol lone. The loss of weight of the solution was 0.8759 g and of the alcohol 0241g.Calculate the molecular weight of dissolved substance.
Solution:
![]()
\(=\frac{0.0241}{0.8759+0.0241}\)
\(=\frac{0.241}{0.9000}\)
According to Rault’s Law for dilute solutions, we get-
\(\frac{P-P_{s}}{P}=\frac{n}{N}=\frac{w M}{w M}\)
w = 3.5, M = Mol. wt. of C2 H5 OH = 46. W = 100, m = ?
Substituting these values in Rault’s Law eqn., we get
\(\frac{0.0241}{0.9000}=\frac{3.5 \times 46}{m \times 100}\)
∴ m = \(\frac{3.5 \times 46 \times 0.9}{100 \times 0.0241}\)
∴ The Mol. wt. of the dissolved substance = 60.13.
Question 16.
At 20°C the vapour pressure of ether is 442 m.m. when 6.1g of Benzoic acid are dissolved in 50g of ether, the vapour pressure falls to 410 m.m. What is the molecular weight of Benzoic acid?
Solution:
According to Rault’s law-
\(\frac{P-P_{S}}{P}=\frac{n}{N}\) (For dilute solutions)
\(=\frac{w M}{W m}\)
where w = wt. of solute = 6.1 : p = 442 m.m.
W = wt. of solvent = 50 : Ps = 410 m.m.
M = mol. wt. of solvent = 74 : P = Ps = 442 – 410
m = mol. wt. of solute = ?
Subtituting these values in the eqn. we have-
\(\frac{32}{442}=\frac{6.1 \times 74}{m \times 50}\)
or,
m = 124.7
Question 17.
Dry air was drawn in succession thought a series of bulbs containing 4.27g of a substance X in 52.68g of ethyl alcohol and through similar set of bulbs containing pure alcohol, the indrawn air and the two sets of bulbs were at the same ‘constant temperature. The. loss of weight in first series of bulbs was 1.292g. and in second series .0313g. Calculate the mole wt.of X.
Solution:

\(=\frac{.0313}{0.0313+1.292}\)
\(=\frac{0.0313}{1.3233}\)
According to Rault’s Law eqn :
\(\frac{P-P_{S}}{P}=\frac{w M}{W m}\)
w = 4.257 g,W = 52.68g, M = 46(mol. wt. of C2H5OH)
Substituting these values in the above eqn.
\(\frac{0.0313}{1.3233}=\frac{4.257 \times 46}{m \times 52.68}\)
∴ m = \(\frac{4.257 \times 46 \times 1.3233}{0.0313 \times 52.68}\)
= 157.1
Question 18.
Air was drawn through a solution containing 38g of solute in 100g of water and then through water. The loss of weight of water was .0551 g and the total weight of water absorbed in sulphuric acid tube was 2.2117g. Find the molecular weight of the dissolved substance.
Solution:
\(\frac{P-P_{s}}{P}=\frac{w M}{m W}\)
\(\frac{0.0551}{2.2117}=\frac{38 \times 18}{m \times 100}\)
∴ m = \(\frac{38 \times 18 \times 2.2117}{.055 \times 100}\)
= 274.5
Question 19.
Dry air was passed through a solution containing 50g of a solute in 100g of water and then through water. The loss in weight of water is 0.05g. The wet air then passed through sulphuric acid whose weight increased by 2.00g. What is the molecular weigh t of the dissolved substance.
Solution:
p – ps = \(\frac{w M}{m V} \cdot \frac{.05}{2.00}\)
\(=\frac{50 \times 18}{m \times 100}\)
∴ \(=\frac{50 \times 18 \times 2.00}{0.5 \times 100}\)
= 360
12th Chemistry Numericals Important Questions with Solutions
Elevation of Boiling Point
Question 20.
The boiling point of a solution Of 3.40 gms. of BaCl2 (Mol. wt. 208,4) in 100 gms. of water is 100.208°C. Calculate the degree of dissociation BaCl2 if the molecular elevation for water is 5.2.
Solution:
Calculation of observed mol. wt.
m = \(\frac{100 \times K \times w}{\Delta T \times W}\)
\(=\frac{100 \times 5.2 \times 3.4}{280 \times 100}\)
= 85.0
But calculated mol.wt. = 208.4
![]()
Particles after dissociation
1 – x + x + x + x = 1 + 2x
But
\(\frac{\text { Normal mol. wt. }}{\text { observed mol. wt. }}=\frac{1+2 x}{1}\)
\(=\frac{208.4}{85}\)
∴ x = 7259
∴ Degree of dissociation of BaCl2 = 72.59%
Question 21.
1.23 g of a substance dissolved in 10g of water raised the boiling point by 0.39″C. Calculate the molecular weight (K = 5.2°C)
Solution:
We know that:
m = \(\frac{100 \times K \times w}{\Delta T \times W}\)
\(=\frac{100 \times 5.2 \times 1.23}{10 \times 3.9}\)
∴ Mol. wt. of the substance =164
Question 22.
1.55 g of urethane dissolved in 34.22g of methyl alcohol raised the boiling point by 0.23″. The elevation constant for methyl alcohol is 8.8. Calculate the molecular weight of urethane.
Solution:
As given in the question, we have-
w = 1 .55 g Δt = 0.32°
W = 34.22g = 8.8
Now m = \(\frac{100 \mathrm{Kw}}{\Delta \mathrm{TW}}\) = \(\frac{100 \times 8.8 \times 1.55}{0.32 \times 34.22}\) = 124.6
∴ Mol wt. of urethane = 124.6.
Question 23.
If the boiling point of water is raised by 0.071° by dissolving 1.17 g of cane sugar (C12 H22O11) in 25g of the solvent, find out the molecular elevation of boiling point
Solution:
As given in the question,
w = 1.17 gm ΔT = .071°
W = 25 gm m = mol. wt. of cane sugar
= 144 + 22+ 176 = 342
Now
m = \(\frac{1000 \mathrm{Kw}}{\Delta T W}\)
K = \(\frac{m \times \Delta T \times W}{100 w}\)
\(=\frac{342 \times 0.071 \times 25}{.1000 \times 1.17}\) = 5.18
Hence the molecular elevation of boiling point = 518
12th Chemistry Numericals Important Questions with Solutions
Question 24.
The boiling point of a solution of 0.105g Of substance in 7.92g of ether was higher than that of the pure solvent by 0.2°. Find the mol wt. of the solute (Kb = 21.1)
Solution:
We know that-
m = \(\frac{100 \times K \times w}{\Delta T \times W}\)
\(=\frac{100 \times 21 \cdot 6 \times 0 \cdot 105}{0 \cdot 2 \times 7 \cdot 92}\)
= 139.8
∴ Mol, wt. of the substance = 139.8
Depression in Freezing Point
Question 25.
Calculate the molar depression constant of water.
Solution:
Latent heat of fusion (LJ) = 80.0 Cal/g
Freezing point of water (T) = 0°C = 0 + 273 = 273°A
∴ K = \(\frac{R T^{2}}{1000 L_{f}}\)
\(=\frac{2 \times(273)^{2}}{1000+80}\)
= 1.863°
= 1.863°
12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions
12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions 12th Chemistry Numericals Important Questions with Solutions